eGFR – 10 years on from the KDIGO Global Recommendations
Graham RD Jones1
1Chemical Pathologist, SydPath, St Vincent’s Hospital Sydney and University of NSW, Australia
Graham.jones@svha.org.au
APFCB News Volume 1, Issue 2
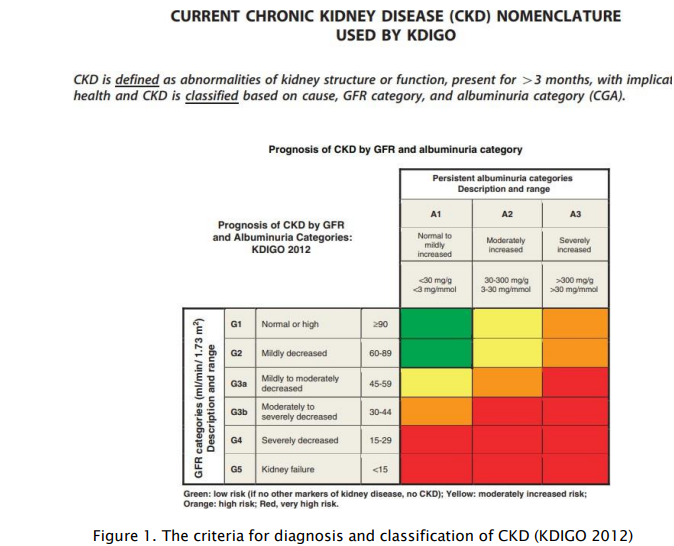
What is GFR?
The GFR is the amount of fluid passing through the combined 2 million nephrons in a person’s kidneys in a period of time. A typical value in a healthy young person is about 100 ml/min which equates to about 144 L per day, of which about 99% is resorbed in the tubules, the remaining excreted as urine. The kidney has many homeostatic functions including waste removal, endocrine, water, electrolyte and acid-base balance and red cell production, and disorders related to these functions are seen as kidneys become damaged. The detection of these signs of CKD (usually by other laboratory tests) however are not used to diagnose or grade kidney function. GFR by contrast provides a single excellent measure of kidney function, irrespective of the cause of the kidney damage, and, importantly, reduction in GFR can be identified at the pre-clinical stage, with the aim of preventing or reducing further damage. GFR also provides a tool to monitor progress and predict possible need for dialysis. An additional vital use for GFR is for drug dosing decisions for renally excreted medications.
GFR can be measured directly, often referred to as a formal GFR test. This type of testing involves intravenous injection, collection of multiple urine or serum samples over several hours, specific analytical techniques (eg measurement of radioactivity for radiolabelled markers) and experience in performing the test. While an estimate of GFR (eGFR, see below) is used almost universally in place of a formal GFR measurement, formal GFR testing is the gold standard for assessing GFR and has an important role for some patients when an accurate measurement is required and eGFR does not suffice, e.g. extremes of body composition, some drug dosing decision (eg some cytotoxic medications), kidney replacement therapy living kidney donors.
What is eGFR?
As GFR is so hard to measure in routine practice there have been developed many equations to estimate GFR in simple practical ways. Historically the Cockcroft and Gault equation, from a single study in 1979, was widely used. .The KDIGO guidelines recommend the use of the CKD-EPI creatinine equation developed in 2009 (CKD-EPI (Cr, 2009)). Alternate equations should only be used if they have been shown to improve accuracy compared with this equation. Like most eGFR equations, CKD-EPI (Cr, 2009) has the inputs of serum creatinine, patient age and sex. Additionally there are versions for African Americans and nonAfrican Americans. With the exception of the race variable a major benefit of this equation is that the inputs are known by the testing laboratory and the formula can be calculated and, as recommended by KDIGO, should be routine reported along with serum creatinine in adults.
Limitations of eGFR
All eGFR equations have limitations. A common assessment of these equations is the percent of eGFR results which are within +/- 30% of a simultaneously measured formal GFR (P30). The best performance that can be expected is a P30 of about 85% ie that for more than 15% the equation may be wrong by more than +/- 30%. In addition, there are factors in the patient and factors in the creatinine measurement that can make the estimate more likely to be wrong. In the patient these can include extremes of muscularity (high or low), pregnancy, dialysis, diet (cooked meat) and sex change. In the creatinine measurement these include assay bias, imprecision and interferences.
Laboratories and eGFR
It is important for laboratories to provide high quality creatinine assays. The key factor to avoid assay bias is traceability to agreed reference standards, usually summarised as IDMS (isotope dilution mass spectrometry) traceability.
A more specific statement would be traceability to reference materials through a reference method in a reference measurement service with all of these components listed on the Joint Committee for Traceability in Laboratory medicine (JCTLM) database. This traceability must be provided through manufacturers to ensure the accuracy of results in laboratori4es using their assays. The other practical factor is the use of enzymatic assays rather than Jaffe assays if possible. This reduces interferences and generally has lower bias and imprecision. Laboratories must also select the eGFR equation to use and most importantly should work with other local or regional laboratories to report in the same way to avoid patients getting difference diagnoses at different laboratories.
The Race-Neutral CKD-EPI equation
Recent work in the United States has challenged the use of race as a health determinant. This is due to poor definitions of race, the risk of race-based discrimination as well as recognising that the concept of race as a social concept not a physical standard. With this in mind, the original CKD-EPI (Cr, 2009) equation was revised in 2021, using the original data, but without a race factor. Using this equation, known as the CKD-EPI(Cr,2021), or race-neutral equation, subjects previously tested using the non-African American equation will have higher eGFR values, by about 5% on average, and those previously assessed with the African American version will have lower results with the new equation.
The National Kidney Foundation in the USA has recommended the immediate uptake of the CKD-EPI (Cr, 2021) equation in the United States. It is unclear what action will be taken in other countries. For individual patients current using the non-African American equation, a 5% increase in eGFR is not highly significant against a background uncertainty of the equation of +/- 30%. There would however be a reduction in the number of people with a diagnosis of CKD, especially in the elderly. There may also be some changes in drug dosing decisions and changes seen when monitoring patients over time. A personal opinion would be that each country should consider this issue and decide for or against changing and ensure uniformity amongst testing laboratories.
Cystatin C
Creatinine based eGFR equations are by far the most widely used globally in clinical practice with creatinine assays being widely available and amongst the cheapest chemistry tests. A limitation to creatinine is that it is produced from muscle and thus differences in the amount of muscle between subjects is a confounding factor. Cystatin C is produced from all cells and thus does not have the same relationship to muscularity that is seen with creatinine. It has also shown less variation between African-Americans and non-African-Americans in the CKD-EPI data. The CKD-EPI (2012, cystatin C) equation does not include a race factor, and its use is being specifically promoted in the USA to avoid the possible effect of race in that setting.
The use of this equation, rather than the CKD-EPI(Cr,2021) race neutral equation increased the P30 from 86% to 89%. An improvement, but not a solution to the wide variability seen with GFR estimating equations. The costs of cystatin assays remain very high compared with creatinine assays and, again as a personal opinion, I think the first action for laboratories is accurate creatinine assays before considering introduction of cystatin C assays.
Drug dosing decisions
This is a vital aspect of the use of eGFR results as many renally-excreted drugs require reduced doses in kidney disease. The best equation for GFR estimation for this purpose has been widely debated over the last 15 years, with the key players being the Cockcroft and Gault equation (C&G) and eGFR, initially with the MDRD equation and now with CKD-EPI. A key factor in this debate is the units used for these tests and the meaning for the difference. C&G is reported in mL/min and CKD-EPI is reported in mL/min/1.73m2. The “1.73m2” factor is an adjustment for a standardised body surface area (BSA). The use of the BSA normalised result is clearly useful for CKD diagnosis and staging, as kidney size, and therefore GFR, is related to the size of the person. Without the BSA normalisation, smaller people (with lower GFRs in mL/min) would be diagnosed with CKD more frequently than larger people, and vice versa. By contrast, for drug dosing, the rate at which a drug is lost from the body in urine depends on the actual amount of fluid passing through the glomerulus (mL/min) rather than a value adjusted for body size.
While C&G reports in mL/min and was widely used in original pharmacological studies, it was developed in only a small number of subjects most of whom were male, using a creatinine assay which is no longer available. Use of this equation also requires the doctor to obtain the patient’s weight and remember to perform the calculation to determine the effect on drug dosing. By contrast the CKD-EPI equations correlate better with the gold standard of measured GFR, and can be readily available on the pathology report. It may however be necessary to remove the inbuilt BSA normalisation, at least in patients markedly larger or smaller than average.
The future
There is ongoing research to try and improve GFR estimating equations. Multiple factors have been considered including measures of body size and composition. While it makes sense, especially for creatinine-based equations, that inclusion of factors related to muscle mass would be an advantage, the improvements have generally been small. Importantly, the difference between research and clinical practice must be recognised and any possible revised equation must be tested in a wide range of subjects (age, body composition, size, diet, physical activity etc) before being considered for use. While this commentary focusses on eGFR, the need for accurate and widely available assays for urine albumin and creatinine is also required for best implementation of CKD testing.
As well as seeking improvements in what is possible with new assays or new equations, the full implementation of current best practice in all laboratories remains an important goal with standardised creatinine assays, use of the same GFR equations and supportive education developed together with renal physicians being vital for patient care. In short, 10 years later, the 2012 KDIGO guidelines remain highly relevant for laboratory management of CKD.
Selected additional reading KDIGO.
Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements 2013;3:3-150. kdigo.org/wpcontent/uploads/2017/02/KDIGO_2012_CKD_GL.pdf
Kidney Health Australia. Chronic Kidney Disease (CKD) Management in Primary Care. 4th edition 2022. https://kidney.org.au/uploads/resources/CKD-Management-inPrimary-Care_handbook_2020.1.pdf
Joint Committee for Traceability in Laboratory medicine (JCTLM). www.jctlm.org/ Inker LA, Eneanya ND, Coresh J. et al. New Creatinine- and Cystatin C–Based Equations to Estimate GFR without Race. NEJM 2021;385;1737-49 (available on-line at NEJM website)
National Kidney Foundation Laboratory Engagement Working Group Recommendations for Implementing the CKD-EPI 2021 Race-Free Equations for Estimated Glomerular Filtration Rate: Practical Guidance for Clinical Laboratories. Clinical Chemistry. 2022:68:511-520. (available on-line at Clinical chemistry website)
Stefani M, Singer RF, Roberts DM. How to adjust drug doses in chronic kidney disease. Aust Prescr 2019;42:163–7. How to adjust drug doses in chronic kidney disease - Australian Prescriber (nps.org.au)