Autoverification in Clinical Biochemistry in an Indian Cancer care set up: Implementation and achievements.
Subhosmito Chakraborty1
1Senior Consultant Department of Biochemistry Tata Medical Center, Kolkata, India
subhosmito@gmail.com
APFCB News Volume 1, Issue 2
ISO 15189 (2012) [4] and the 112 documents from the National Accreditation board for Laboratories (NABL) provide an overarching and very broad set of rules. The AUTO10-A (CSLI) adds some specificity and defines Boolean logic and algorithm development in some details [3]. There is, however, a void of guidelines on the specifics of auto verification. This task is daunting if not impossible because the requirements of each hospital or stand-alone laboratory is different. Our laboratory is different in demographics and medical characteristics as it caters to a cancer population in a tertiary set up.
Preplanning
In preplanning, we deeply introspected our need for an AV system. Our management was convinced of the need for an AV system. The goal was uniformity in result evaluation, role-based access to staff, reduced fatigue across all levels of personnel, reduced manual record keeping, improved turnaround times (TAT), better utilization of staff, and reduction of laboratory errors Vendors for AV systems should be carefully selected to meet the required goals. They should train personnel and provide maintenance and support services. We selected Instrument Manager® (IM) from Ortho Clinical Diagnostics (Ortho) as our AV system.
This could directly link with the in-house laboratory informatics system (LIS) and connect to the XT-7600 analysers (Ortho). A primary and a back-up computer were provided for the task. Agreements were made with the vendor in securing the goals. Both parties agreed that rules would be developed in-house and the medicolegal onus of the rules will lie with the laboratory.
Team Building
The information technology (IT) team, the laboratory technical team, and a team of the signatories were organized. They were primed to initiate, implement, and maintain the AV system. The IT team collaborated with Ortho for procuring and structuring the hardware and software systems. The technicians’ team were trained in a phased manner to accustom to them to the IM and wean off their total dependence on the LIS system of seven years. The signatories’ team were entrusted to build the algorithms and chalk out the validation and verification of the systems as per the ISO (Clause 5.9) and NABL guidelines. Figure 1 shows an algorithm of serum sodium validation for both outpatients and hospitalized patients.
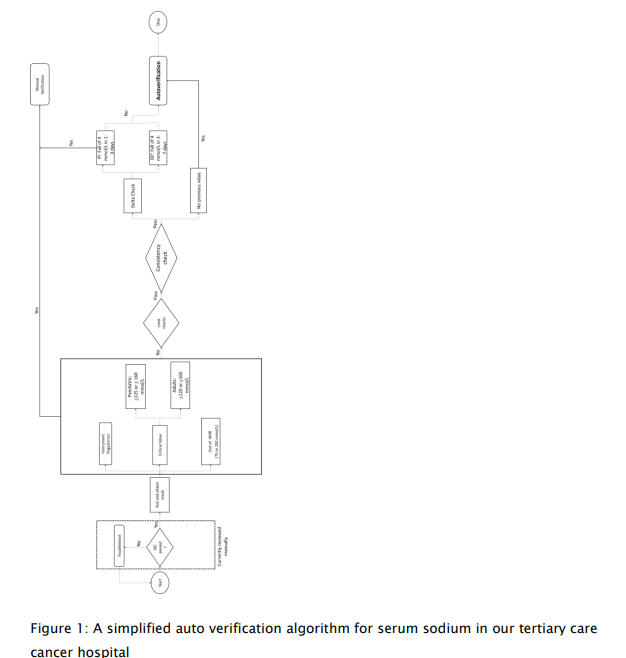
Development of computer logic
The computer Boolean logic was based on instruments flags and warnings, delta value checks, stochastic derivation of limit checks (review intervals), critical values, consistency checks, and other customized checks (gender and age). Internal quality controls (IQC) were deliberately kept in manual review as non-departmental staff were also involved in running IQC due to their involvement in shift and holiday duties. A detailed guide can be found in the paper by Randel ET. Al [5]. Validation and verification were carried out in the IM itself as it had two distinct segments for rules – “testing” and “go-live” environments. The rules were first tested on simulated data and then real patient data in the testing environment. Records were kept as per the guidelines.
Risk management for the AV system
A risk management strategy was developed wherein the back-up computer could function as the primary computer or an entire shift to the LIS system in case of breakdown. Several trials were given by shutting off the primary computer during off-peak hours. Caution needs to be exercised in routine AV operations. The rules are of if-then-else type and cannot always predict errors in complex situations. Some rules may inadvertently interact with others. Software upgrades must need a fresh set of validation or verification. It is difficult to pre-judge all such scenarios beforehand. So, the learning curve on an AV system is continuous and daily supervision is a must.
Results
We went live with AV in October 2017 with the metabolic panels after prior intimation to our clinicians. We actively sought inputs to abnormal results. Auto validated reports were marked as “Auto verified”. Intensive audits which were performed to check for outliers and inadvertent auto validated results for three consecutive months. The audits are currently performed on ten random samples a day. A provision has been kept to increase the number of audits if test methods or the AV rules change.
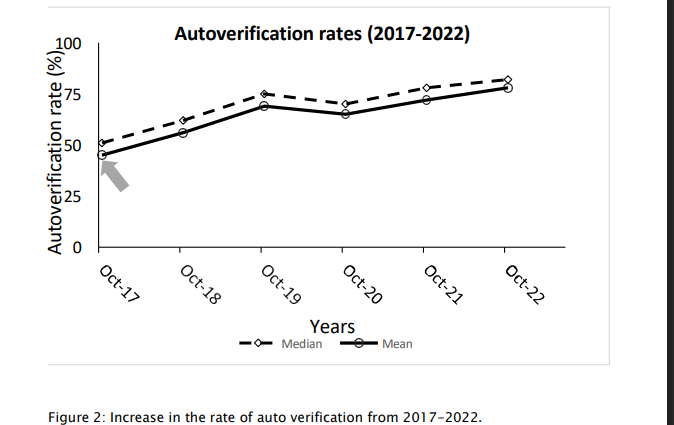
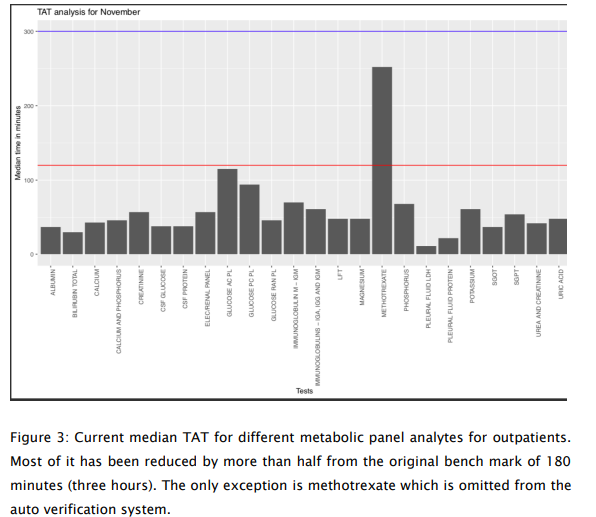
The AV system along with the track system (from Ortho) proved to be a gamechanger. 78% of the metabolic panel tests are currently auto validated (Fig 2). Manual tasks were reduced by 50.2% for the technologists. Unnecessary repeat blood draws were avoided in up to 7% of the samples due to visible hemolysis, turbidity, or icterus. Pre-analytical errors could be detected in up to 10% of the samples. This group could now focus on communication of critical results, writing operating procedures and so on. Thus, this led to better staff utilization. TAT which were fixed at three hours after sample receipt in the laboratory were reduced by 50-60% (Fig. 3). Now, we can deliver results within 1.5 hours for general patients and 52-58 minutes for the intensive care and day-care chemotherapy sections. Signatories could now focus on the absolutely critical results and subsequent discussions and communications to clinicians.
Conclusion and future directions
Our future plans are pivoted on specialty-based reports such as those of surgical oncology, haemato-oncology, medical oncology, or paediatric oncology. We also intend to use the moving averages for selected parameters and incorporate daily IQC runs as a part of AV. Overall, we and our clinical colleagues are satisfied with the operation of the system in place. Rarely, there has been a complaint on the AV system that has come to our notice. Most problems (98%) have been identified to have arisen in the pre-preanalytical and pre- analytical phase and are out of direct control of the laboratory. To summarize, implementation of the system requires forethought and scrupulous planning, developing logic, frequent audits, clinical communications and collaborations, and daily monitoring. It is indeed satisfying to have an AV system in place for a busy 24 x 7 hospital based laboratory.
1. Feitosa SM. et al., J Bras Patol Med Lab, 2016; 52: 149-156
2. Li J. et al., Ann Clin Biochem., 2018; 55(2); 254-63
3. Wayne PA, Clinical and Laboratory Standards Institute, CSLI document AUTO10- A; 2006
4. Medical Laboratories-Requirements for Quality and Competence, ISO 15189: 2012
5. Randell E. W. et al., Clin Biochem., 2019; 73: 11-25
