Hemoglobin A1c: Summary of Existing Test Methods and Introduction of a Novel Assay Design
Ted DiMagno1, Lily Li2
1,2Conflict of Interest: All Authors declare no conflict of Interest Content Owner: Ortho Clinical Diagnostics Source of funding: Ortho Clinical Diagnostics.
E-mail: shannon.voon@orthoclinicaldiagnostics.com
APFCB News Volume 1, Issue 2
Chronic hyperglycaemia results in a greater abundance of circulating glycated proteins, which play an important role in the pathophysiology of diabetes.4 Of particular importance is glycated hemoglobin A1c (HbA1c), which is an indicator of average blood glucose concentration over the previous 3 months.5,6 In the 1990s, two landmark clinical trials, the Diabetes Control and Complications Trial (DCCT) and the UK Prospective Diabetes Study (UKPDS) demonstrated that controlling hyperglycemia, as assessed by serum HbA1c, led to a reduction in diabetes complications.7–10 As a result, HbA1c measurement has become the gold standard for diabetes diagnosis and monitoring.5, 11 Here we review diabetes diagnosis and monitoring, the pathological impact of chronic hyperglycemia, and the molecular features of HbA1c relevant to diabetes pathophysiology and HbA1c measurement. We also highlight international efforts to standardize HbA1c measurement, as well as current HbA1c testing methods, including a new and innovative Dry Slide HbA1c assay.
Diabetes Disease Burden
Epidemiology
Diabetes is a major cause of morbidity and mortality worldwide.2 In a large cohort study including >1 million individuals from 7 Asian countries, those with diabetes had a nearly 2-fold risk of all-cause mortality compared with those who did not have diabetes.12 individuals with diabetes worldwide is projected to increase from 537 million adults in 2021 to 783 million by 2045.2 This increase will be largely driven by an increase in type 2 diabetes due to higher rates of obesity and more sedentary lifestyles. Over the same time period, the worldwide direct costs of diabetes—currently 11.5% of total healthcare costs—are estimated to increase from $966 million to $1.05 trillion USD.2 Clinical pathology
The term diabetes refers to a class of metabolic disorders that is usually divided into two main categories.1 Type 1 diabetes makes up a small percentage of diabetes cases (5%–10%) and is caused by autoimmune destruction of pancreatic β cells that impairs insulin production and secretion. The vast majority of diabetes cases, however, are type 2 diabetes (90%–95%), which is characterized by insulin resistance rather than insulin insufficiency. Whereas individuals with type 1 diabetes require daily insulin injections to manage their hyperglycemia, those with type 2 diabetes generally do not, at least initially.1 Management of type 2 diabetes instead usually consists of lifestyle modifications, including healthy diet and exercise, with an emphasis on maintaining a healthy weight, although medications may also be used if lifestyle change alone is not enough to control the hyperglycaemia.
More than 80% of end-stage renal disease is caused by diabetes, hypertension, or a combination of the two.13 One systematic literature review concluded that the prevalence of cardiovascular disease in patients with type 2 diabetes was 32%, and that cardiovascular disease caused approximately half of the deaths observed in the studies reviewed.14 In a separate pooled analysis of data from 22,896 diabetic individuals, the prevalence of diabetic retinopathy was 35%, and the prevalence of vision-threatening diabetic retinopathy was 10%.15 Another, smaller study found a prevalence of 26% for painful diabetic peripheral neuropathy.16 These and other data highlight the significant burden of diabetes-related complications on individuals with the disease. Multiple pathophysiological mechanisms contribute to the development of diabetes complications.1 Perhaps chief among these is oxidative stress.4,17,18 The term glycoxidative state has been used to describe the persistent environment of oxidative stress due to chronic hyperglycemia that underlies many of the pathological effects of diabetes.4,17 Oxidative stress contributes to damage and dysfunction of the vascular endothelium through multiple mechanisms and is thus associated with both the macrovascular (coronary artery disease, peripheral arterial disease, and stroke) and microvascular (diabetic nephropathy, neuropathy, and retinopathy) complications.10, 13,18 Higher levels of protein glycation under hyperglycemic conditions contribute to the increase in oxidative stress by promoting formation of early and advanced glycation end products (AGEs), leading to the generation of free radicals and oxidants.4 HbA1c is not only an indicator of average blood glucose levels over the long term, it is also an early glycated protein that can undergo further chemical modification to generate hemoglobin-AGE, which may contribute to vascular endothelial dysfunction by blocking nitric oxide production. HbA1c itself may enhance oxidative stress, since the glycated protein is more susceptible to digestion by endogenous proteases, a process that releases heme, ferrous iron, and free radicals.4
Clinical Use of HbA1c Testing
Why and when to measure HbA1c
Undiagnosed diabetes is of particular concern as chronic hyperglycemia can lead to microvascular and macrovascular damage, causing more severe complications and a higher risk of death the longer the condition goes untreated.1,2 Early diagnosis of prediabetes and diabetes, followed by aggressive lifestyle modifications, medical treatment, and close monitoring is key to improving quality of life and reducing mortality risk.2,19
The American Diabetes Association (ADA) recommends routine screening of low-risk individuals every 3 years starting at age 45 and more frequent screening for high-risk asymptomatic individuals (e.g., smokers and those suffering from obesity or hypertension) and patients with prediabetes.1 Patients with diabetes should have their glycemic status monitored using HbA1c at least twice a year if they are meeting their glycemic goals and at least every 3 months if those goals are not being met or if there has been a change in therapy.19
Current diagnostic criteria for diabetes
According to ADA guidelines, diabetes may be diagnosed either by measuring HbA1c or plasma glucose.1 Acceptable diagnostic criteria include one of the following:
1. HbA1c ≥6.5% measured by a National Glycohemoglobin Standardization Program (NGSP)–certified method standardized to the DCCT assay
2. Fasting plasma glucose ≥126 mg/dL (≥8 hours fasting)
3. Oral glucose tolerance test (OGTT) ≥200 mg/dL (2-hour plasma glucose)
4. Random plasma glucose ≥200 mg/dL with symptoms of hyperglycemia/hyperglycemic crisis
Unless the patient is exhibiting classic symptoms of hyperglycemia or is experiencing a hyperglycemic crisis, an abnormal screening result based on criteria 1–3 must be confirmed with a second test, either using the same or a different testing method
Advantages of HbA1c testing compared with fasting plasma glucose include greater patient convenience with no need for fasting, better preanalytical sample stability, less short-term variability in marker levels, and assay standardization.1 Disadvantages of HbA1c testing include a higher cost compared with plasma glucose measurement and potentially limited availability in the developing world, although access continues to improve. In addition, HbA1c should not be used for certain patient populations, including those with conditions that affect the red blood cell (RBC) lifespan (normally ~120 days) such as pregnancy, recent blood transfusions, or HIV treatment.20 It should also not be used with patients who have interfering levels of genetic hemoglobin variants or chemically modified hemoglobin derivatives.5,6,20–22 In these cases, alternative methods should be considered, such as plasma glucose testing or measurement of fructosamine, glycated serum protein, or glycated albumin. The glycated protein tests provide a shorter glycemic view of 2–3 weeks compared with 3 months for HbA1c testing, which can also be beneficial when monitoring the impact of changes in treatment.5,6 Monitoring glycemic control
As mentioned above, patients with diabetes require routine monitoring of glycemic status to assess therapeutic effectiveness and determine if there is a need for further intervention.19 When setting HbA1c target goals, the physician should consider individualized needs based on patient lifestyle and health risks (such as diet and exercise, patient’s age, disease duration, or existing comorbidities). While HbA1c <7% is a suitable goal for many, a lower cut-off may be appropriate if it is safely achievable for the patient.19 Alternatively, a higher cut-off may be necessary for patients with certain medical conditions or whose HbA1c remains somewhat elevated despite receiving standard of care disease management. Other vascular and metabolic parameters such as blood pressure and serum lipids should be monitored to assess treatment efficacy and determine if any changes are required. In addition to routine laboratory HbA1c measurement, it may be desirable for the patient to frequently selfmonitor glucose levels to adjust behavior and daily insulin levels. Recent advances in technology such as continuous glucose monitoring (CGMs) have made blood glucose monitoring simpler and more informative.19
HbA1c Structural Biology
Chemical structure and glycation reaction
Haemoglobin A (HbA) is the primary form of haemoglobin in adults, accounting for approximately 97% of circulating hemoglobin.11 HbA is a heterotetramer consisting of two identical α chains and two identical β chains; each of the four chains has a globular structure that surrounds a heme group containing a single iron atom. The main function of HbA and other forms of haemoglobin is to transport oxygen through the body.23
Although there are multiple forms of glycated HbA, HbA1c, in which glucose is added to the N-terminal valine residue of the HbA β subunit to form fructosyl valine, accounts for approximately 80% of glycated HbA in the human bloodstream (Figure 1).11 HbA1c is formed nonenzymatically through a Maillard reaction in which a glucose molecule forms a Schiff base with the valine residue.24 The Schiff base then undergoes an Amadori rearrangement, creating a nonreversible covalent bond. The stability of the HbA1c protein–glucose adduct makes it a useful indicator of average blood glucose levels over time, given that HbA1c levels are directly correlated with average blood glucose concentrations.5,11 Hemoglobin is found in RBCs, which normally have an average lifespan of 90 days; thus, HbA1c levels reflect blood glucose levels over the previous 3 months.5,6
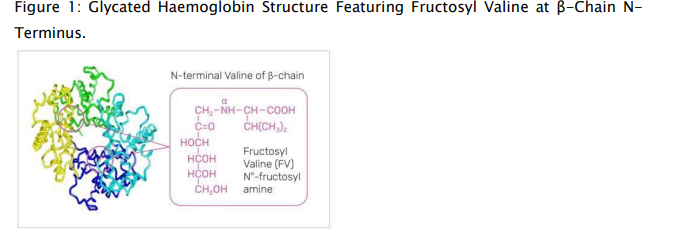
In addition to the other, less plentiful forms of glycated HbA, glucose can also glycate other serum proteins, including albumin.4 Therefore, measuring other glycated proteins may be a suitable alternative to HbA1c testing in patients who have altered RBC lifespan or hemoglobin variants.
Sources of HbA1c measurement interference
As with any clinical laboratory test, HbA1c measurement is subject to multiple types of interference, including hemoglobin variants.5 Structural variants of HbA are caused by point mutations in the genes encoding the protein’s subunits, resulting in amino acid substitutions that can alter hemoglobin structure and, potentially, function.25 When individuals are homozygous for a genetic hemoglobin variant, they may develop a symptomatic disease, e.g., sickle cell anemia in HbS homozygotes. HbA1c testing is not appropriate for these patients.5 On the other hand, individuals who are heterozygous for a hemoglobin variant may not be phenotypically different from individuals who are homozygous for HbA (non-variant hemoglobin). HbA1c testing may be appropriate for patients who are heterozygous for a hemoglobin variant, depending on the testing method.5,6,21
The worldwide prevalence of hemoglobin variants is 5%–7%, with four single amino acid substitution variants, namely HbS, HbE, HbC, and HbD, being the most common.25 Position 1 on the HbA β chain is the N-terminal valine residue that is the glycation site targeted by a number of HbA1c assay methods.5,25 HbS, the hemoglobin variant that causes sickle-cell anemia, has an amino acid substitution (valine for glutamic acid) at position 6 on the β chain, whereas the variant HbC has a different substitution (lysine for glutamic acid) at the same position. HbE has a lysine for glutamic acid substitution at position 26, and HBD has a glutamine for glutamic acid substitution at position 121.25
The prevalence of hemoglobin genetic variants can vary by geography.5 For example, approximately 300 million people worldwide are heterozygous for HbS, with parts of Africa, the Middle East, and India having HbS allele frequencies >5%.5,26 The highest prevalence of HbC, 40% - 50%, is seen is parts of West Africa.27 HbD is most prevalent (2% - 3%) among Sikhs in the Punjab region of India and is also found in many individuals in Northwest India, Pakistan, and China.28 HbE is particularly prevalent in South-East Asia, being present in 30% - 40% of the population in some regions.29 For patients with these hemoglobin variants, an HbA1c testing method that is unaffected by hemoglobin variants is recommended.25 Elevated fetal hemoglobin (HbF) can interfere with some HbA1c assays.25 Although HbF is the major hemoglobin species in fetuses, it usually accounts for <1% of circulating hemoglobin in adults. Some individuals, however, are genetically predisposed to persistently elevated HbF levels in adulthood, a condition that is asymptomatic in many cases. Elevated HbF is also associated with certain medical conditions, such as multiple myeloma.25
In contrast to the physiological interference associated with anemia caused by hemoglobinopathies and other conditions, analytical interference can be caused by structural/biochemical changes due to amino acid substitutions.11 Substitutions that change the net ionic charge of HbA may cause interference with methods that separate molecules based on charge differences, such as ion-exchange high-performance liquid chromatography (HPLC) or capillary electrophoresis.25 Some immunoassay methods may have interference depending on where the detection epitope it targets is located.5 If it is near an amino acid substitution or if the amino acid substitution results in a protein conformation that inhibits access to the epitope, it may interfere in the test measurement. Additionally, mutations at the glycation site may alter the glycation rate, thus affecting the results of immunoassays or boronate affinity HPLC measurement methods.30 Although some HPLC methods do not separate HbF from HbA1c or HbA1, others can separate even elevated levels of HbF from the HbA peaks.25 The N-terminal residue of HbF γ chains (analogous to HbA β chains) is glycine instead of valine, which is likely glycated at a lower rate. Boronate affinity methods, which measure the ratio of glycated to non-glycated hemoglobin, will give an HbA1c result lower than the actual value in patients with elevated HbF.25
Laboratories need to consider the impact of hemoglobin variants on their HbA1c testing methods, particularly when serving patient populations where specific variants are more prevalent.25 As the majority of hemoglobin variants are genetic in origin, they may only need to screen new patients using methods capable of detecting variants and then use easier, higher-throughput testing methods for subsequent HbA1c measurements. Clinicians should also be aware of the limitations of HbA1c testing for patients with specific variants and order tests for variants when they suspect hemoglobinopathy or note discordance between a patient’s HbA1c measurements and his or her self-monitored blood glucose levels.19
Chemical derivates of hemoglobin can also affect the accuracy of HbA1c measurement.11 One such derivative created by labile carbamylation of the N-terminal valine is common in uremic patients. Carbamyl-hemoglobin may interfere with results based on charge, since the two forms of hemoglobin have similar isoelectric points, increasing the reported amount of HbA1c. Schiff-base hemoglobin (an intermediate in HbA1c formation) is another possible source of interference.11
Standardization of HbA1c Measurement
Use of HbA1c as a biomarker of glycemic control was proposed in the early 1990s, spurred in part by results from the DCCT, which demonstrated that controlling HbA1c levels in patients with type 1
diabetes reduced microvascular complications, including diabetic retinopathy, nephropathy, and neuropathy.9 The DCCT was followed by the UKPDS, which demonstrated that lowering HbA1c decreased microvascular and macrovascular (i.e., cardiovascular disease–related) complications in patients with type 2 diabetes, further supporting the use of HbA1c as a marker for diabetes management.7 However, implementation of HbA1c testing was hampered at the time by the high variability in test results.31
To address this variability, two different initiatives were established to standardize HbA1c measurements across testing methods: the International Federation of Clinical Chemistry (IFCC) Working Group on Hemoglobin A1c Standardization and the National Glycohemoglobin Standardization Program (NGSP).32,33 The IFCC Working Group established reference methods for HbA1c analysis to ensure accuracy-based results using primary reference materials made of HbA1c and HbA0 (non-glycated hemoglobin), which are first isolated by cation exchange and affinity chromatography.6,32,34,35 After the proteins are digested by proteolysis, the glycated and non-glycated N-terminal peptides of the hemoglobin β chain are then quantified by either mass spectrometry or capillary electrophoresis.35 The IFCC has a network of approved laboratories and offers calibrators to manufacturers, as well as bimonthly monitoring to ensure traceability back to the IFCC standard.33 The NGSP was established with the goal of standardizing HbA1c test results to those of the DCCT and UKPDS, “which established the direct relationships between HbA1c levels and outcome risks in patients with diabetes.”36 To that end, the NGSP and its network of laboratories work with manufacturers to establish calibration settings for their HbA1c tests, provides annual certification for manufacturers and laboratories, and performs proficiency testing of routine clinical laboratories (Figure 2).33,36
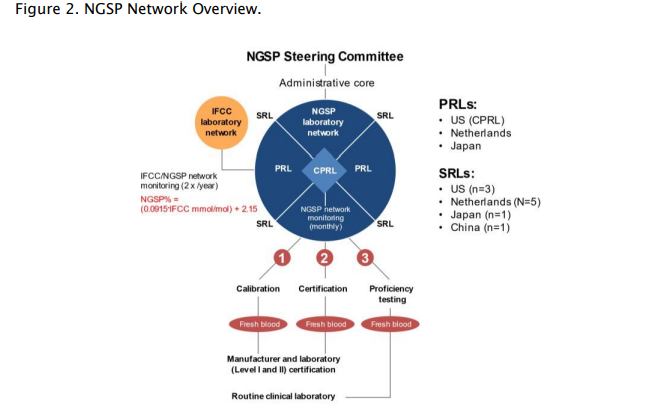
Figure adapted from Little et al. Clin Chem. 2019;65(7):839-848. CPRL: central primary reference laboratory; IFCC: International Federation of Clinical Chemistry; NGSP: National Glycohemoglobin Standardization Program; PRL: primary reference laboratory; SRL: secondary reference laboratory.
Calibration ensures that HbA1c measurements—regardless of method or equipment used—are comparable to DCCT results.33 To achieve this goal, the NGSP provides support to HbA1c test manufacturers for initially calibrating their methods and then confirming that calibration.
NGSP certification, which is valid for 1 year, requires a manufacturer to demonstrate that its method meets specific criteria, which have become more stringent over time (Figure 3). Since 2019, manufacturer certification requires that results from 36 of 40 individual whole blood samples be within ±5% of results for the same samples from a secondary reference laboratory, in a blinded comparison.33,37 To monitor HbA1c values in clinical laboratories, the NGSP assesses the College of American Pathologists (CAP) HbA1c proficiency surveys that use pooled whole human blood.33
Figure 3. CAP Proficiency Demonstrating Standardization Over Time and NGSP Certification Requirements.
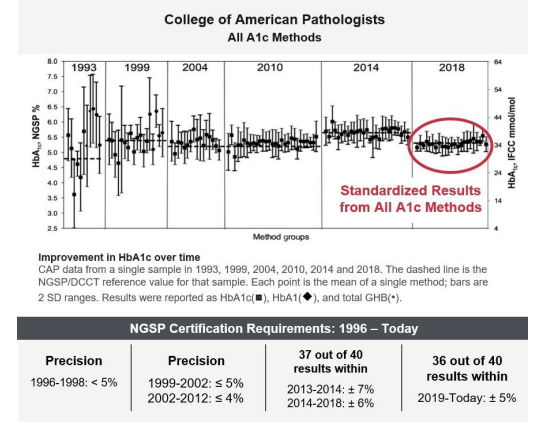
Figure adapted from Little et al. Clin Chem. 2019;65(7):839-848. CAP: College of American Pathologists; DCCT: Diabetes Control and Complications Trial; GHB: glycated hemoglobin; HbA1c: hemoglobin A1c; IFCC: International Federation of Clinical Chemistry; NGSP: National Glycohemoglobin Standardization Program; SD: standard deviation. Because the IFCC method uses purified standards, it is considered a higher order method, in contrast to the designated comparison method used by the NGSP, which is based on measurements from blood samples and is not completely specific for HbA1c.33 The NGSP and IFCC use different measurement units: %HbA1c for NGSP and mmol HbA1c/mol Hb for IFCC. For this reason, a master equation has been developed that describes the relationship between the two standardization systems: NGSP = [0.09148 * IFCC] + 2.152.32 The rigorous work of the NGSP and IFCC provides confidence in HbA1c result standardization, which has contributed to reduced variability of clinical HbA1c measurements over time (Figure 3), thus enabling better diabetes care based on more accurate test results.33
HbA1c Testing Methods
A variety of HbA1c testing methods have been developed over the years, as advances in technology have minimized HbA1c hemoglobin variant interference, increased throughput, and provided other operational advantages.
High-performance liquid chromatography (HPLC)
HPLC methods measure HbA1c by either ion-exchange or boron ate affinity. Ionexchange chromatography separates different forms of hemoglobin according to ionic charge.11 The proteins form ionic bonds with the charge solid phase of the chromatography column and are then eluted based on charge using buffers of increasing ionic strength, which disrupt the binding between the protein and solid phase.6 As proteins are eluted from the column, they are detected, and a chromatogram is generated showing peaks corresponding to each eluted protein species. The concentration of HbA1c is calculated based on these peaks.11 Examining the chromatogram can reveal potential interferences and allow detection of hemoglobin variants, assuming those variants don’t co-elute with either HbA1c or HbA based on charge.25,31 For example, HbF co-elutes with HbA1c using older ion-exchange HPLC methods; newer ion-exchange methods produce a separate peak for normal levels of HbF, but only some of these can also separate peaks for elevated levels of HbF.25 Methods that use high-resolution chromatography decrease the interference from hemoglobin variants and derivatives but also take longer than lower resolution methods, so the tradeoff between more accurate variant detection and time/throughput needs to be considered when choosing a method.5,11
Another HPLC-based method for HbA1c measurement is boronate affinity chromatography.5,11,30 In this method, the column contains a gel bonded to maminophenylboronic acid, which forms a complex with the cis-diol groups of hemoglobin-bound glucose. The glycated hemoglobin is then eluted from the column by adding sorbitol. Because all glycated hemoglobins are detected, this method is largely unaffected by interference from hemoglobin variants. However, this method cannot detect the presence of hemoglobin variants.5
Capillary electrophoresis
Capillary electrophoresis separates molecules by charge—like ion-exchange HPLC—and also by mass.5 This allows identification of hemoglobin variants and other interferences by displaying separated peaks on an electropherogram.30(p49) Given the effective separation of molecules by mass and charge, the most common variants do not produce analytical interference affecting the HbA1c measurements.5 This method can characterize and diagnose hemoglobin variant type for clinicians who are interested in this information.5,30
Immunoassays Immunoassays are antibody-based methods that bind a targeted epitope on the hemoglobin β chain.5,25 Typically these antibodies bind the first 4–10 amino acids of the β chain, and can be subject to interference from the HbS and HbC variants, since the sixth amino acid is substituted and prohibits access due to a structural change in the hemoglobin.5 Downstream amino acid substitutions found in HbD and HbE are further away from the N-terminus and, thus, the epitope can be recognized by the antibody, resulting in little to no interference. The antibodies used in HbA1c immunoassays do not recognize chemically modified hemoglobin derivatives.11 Immunoassays are easy to implement in routine clinical laboratories and are not affected by ionic charge differences in hemoglobin variants or derivatives.38 However, this method cannot detect the presence of hemoglobin variants.
Enzymatic HbA1c testing
Newer HbA1c measurement methods are based on enzymatic detection. In these methods, proteolytic digestion of lysed whole blood results in fragmentation of the HbA1c β chain and release of its N-terminal fructosyl valine, which is detected via a horseradish peroxidase–catalyzed reaction with a chromogen.39,40 Enzymatic methods are not very sensitive to interference from hemoglobin variants because the most common variants are upstream on the HbA1c β chain (Figure 4). However, this method cannot detect the presence of hemoglobin variants.5
Figure 4. Cleavage Site for Hba1c Enzymatic Assay Substrate Relative to Locations of Common Hemoglobin Variant Amino Acid Substitutions.
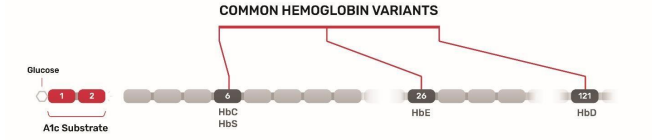
HbC, HbD, HbE, and HbS represent the most common genetic hemoglobin variants. Recently, a Dry Slide method for enzymatic HbA1c testing has been developed: VITROS® Chemistry Products A1c Slides (Figure 5).41(p1) This dry and multilayer slide system features reagents applied to a clear polyester support base cut to the size of a postage stamp. This testing method uses a single drop of neat whole blood that is placed on the top spread layer. The spread layer filters out interferences such as hemoglobin, turbidity, and paraproteins. In this test design, the slide contains two surfactants and a protease. The first surfactant lyses the RBCs, while the second surfactant denatures the glycated hemoglobin released from the cell. The protease cleavage site on the hemoglobin molecule is accessible after denaturation, allowing the protease to cleave a two-peptide fructosyl-alpha-valylhistidine fragment from the N-terminus of HbA1c. Larger hemoglobin fragments and other filtered interferences remain trapped in the spread layer, while the smaller hemoglobin fragments filter through the masking layer until it reaches the reagent layer. In the reagent
layer, the dipeptide substrate is oxidized by a highly specific fructosyl amino acid oxidase to produce hydrogen peroxide, which triggers oxidation of a leuco dye by horseradish peroxidase, producing a colorimetric signal directly proportional to the concentration of glycated hemoglobin. This reaction is detected by reflectance spectroscopy. The masking layer minimizes optical interference to enable accurate results.41(p1)
Figure 5. VITROS® A1c Slides Schematic Showing the Three Functional Reagent Layers.
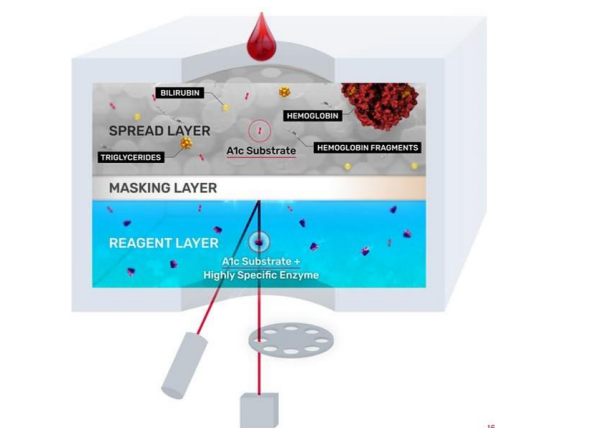
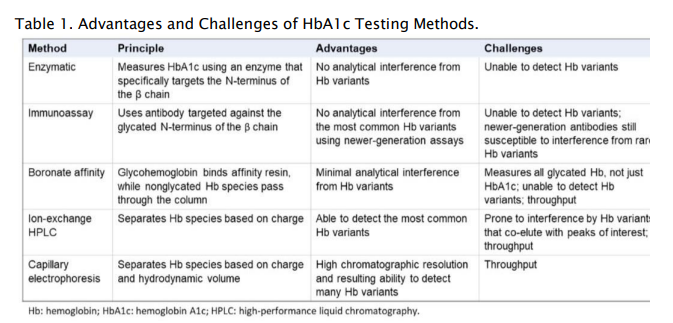
VITROS® A1c Slides are tested on VITROS® Integrated or Chemistry Systems along with other routine and esoteric tests that can optimize lab workflow on a consolidated testing platform.41 With up to 180 tests per hour, this method simplifies whole-blood management with less hands-on time, directs primary test tube sampling, and is compatible with the VITROS Automation Solutions track. VITROS® A1c Slides have excellent performance standardized to the NGSP Tosoh G8 method and are NGSP certified as required by ADA guidelines.1,41,42 As an enzymatic method is used, no clinically significant interference is seen with common haemoglobin variants (HbS, HbC, HbD, and HbE). The Dry Slide format is impervious to reagent degradation and has excellent performance stability and calibration stability up to 20 weeks. Comparing HbA1c testing methods Each of the available HbA1c testing methodologies has its advantages and challenges (Table 1). Test selection should be based on laboratory objectives and testing needs, including need for a diagnostic claim and identification of hemoglobin variants, or avoidance of hemoglobin variant interference. The patient population being tested should also be considered, since hemoglobin variant prevalence differs by geography as well as by racial/ethnic composition. High-throughput platforms may be more suitable for high-volume testing, and multi-test platforms offer operational efficiencies that single-test platforms do not.
Conclusion
The reliability of HbA1c measurements across various assay technologies is ensured by the standardization work of the NGSP and IFCC.33 This standardization links HbA1c measurements to clinical outcomes from early landmark trials demonstrating the relationship between controlling HbA1c levels and the reduction of diabetes-related complications. Easy, accurate, and fast measurement of HbA1c promotes better diabetes care through the assessment of average blood glucose levels over time, enabling diabetes diagnosis and monitoring of glycemic control. Laboratories should consider their own testing objectives (i.e., detection or avoidance of hemoglobin variant interference, test volumes, ease of use) in the selection of HbA1c testing methods. The novel, enzymatic VITROS® A1c Slides assay offers simplified and integrated workflows on a consolidated testing platform with high throughput for routing testing.41 The assay has no clinically significant interference from common hemoglobin variants.
Acknowledgments
Amy Volpert of Bioscience Communications, New York, NY who provided medical writing support for this manuscript. Andrea Ott-Vasconi, BS MBA of QuidelOrtho provided content input.
1. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37 Suppl 1:S81-90. doi:10.2337/dc14-S081
2. International Diabetes Federation. IDF Diabetes Atlas, 10th Edition. Brussels, Belgium. Published 2021. Accessed July 19, 2022. https://diabetesatlas.org/atlas/tenth-edition/
3. World Health Organization. The top 10 causes of death. Published December 9, 2020. Accessed July 19, 2022. https://www.who.int/news-room/factsheets/detail/the-top-10-causes-of-death
4. Saleh J. Glycated hemoglobin and its spinoffs: Cardiovascular disease markers or risk factors? World J Cardiol. 2015;7(8):449-453. doi:10.4330/wjc.v7.i8.449
5. Rhea JM, Molinaro R. Pathology consultation on HbA(1c) methods and interferences. Am J Clin Pathol. 2014; 141(1):5-16. doi:10.1309/AJCPQ23GTTMLAEVL
6. Campbell MR, Shokrani M. Comparison of HbA1c and glycated protein methodologies. Am Soc Clin Lab Sci. 2016;29(2):114-121. doi:10.29074/ascls.29.2.114
7. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352(9131):837-853.
8. The Diabetes Control and Complications Trial Research Group. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968-983. doi:10.2337/diab.44.8.968
9. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. doi:10.1056/NEJM199309303291401
10. Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405-412. doi:10.1136/bmj.321.7258.405
11. Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clin Chem. 2001;(2):11.
12. Yang JJ, Yu D, Wen W, et al. Association of diabetes with all-cause and cause-specific mortality in Asia: A pooled analysis of more than 1 million participants. JAMA Netw Open. 2019;2(4):e192696. doi:10.1001/jamanetworkopen.2019.2696
13. International Diabetes Federation. IDF Diabetes Atlas, 9th Edition. Brussels, Belgium. Published 2019. Accessed July 19, 2022. https://diabetesatlas.org/atlas/ninthedition/
14. Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007-2017. Cardiovasc Diabetol. 2018;17(1):83. doi:10.1186/s12933- 018-0728-6
15. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564. doi:10.2337/dc11-1909
16. Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29(7):1518-1522. doi:10.2337/dc05-2228
17. Piarulli F, Sartore G, Lapolla A. Glyco-oxidation and cardiovascular complications in type 2 diabetes: a clinical update. Acta Diabetol. 2013;50(2):101-110. doi:10.1007/s00592-012-0412-3
18. Rochette L, Zeller M, Cottin Y, Vergely C. Diabetes, oxidative stress and therapeutic strategies. Biochim Biophys Acta BBA - Gen Subj. 2014;1840(9):2709-2729. doi:10.1016/j.bbagen.2014.05.017
19. American Diabetes Association. Glycemic targets: Standards of medical care in diabetes. Diabetes Care. 2022;45(Suppl 1):S83-S96. doi:10.2337/dc22-S006
20. American Diabetes Association. Classification and diagnosis of diabetes: Standards of medical care in diabetes. Diabetes Care. 2022;45(Supplement_1):S17-S38. doi:10.2337/dc22-S002
21. Tavares RS, Souza FO de, Francescantonio ICCM, Soares WC, Mesquita MM. HbA1c levels in individuals heterozygous for hemoglobin variants. Rev Assoc Medica Bras 1992. 2017;63(4):341-346. doi:10.1590/1806-9282.63.04.341
22. Rodriguez-Capote K, Tovell K, Holmes D, Dayton J, Higgins TN. Analytical evaluation of the Diazyme glycated serum protein assay on the siemens ADVIA 1800: comparison of results against HbA1c for diagnosis and management of diabetes. J Diabetes Sci Technol. 2015;9(2):192-199. doi:10.1177/1932296814567894
23. Zur B. Hemoglobin variants – pathomechanism, symptoms and diagnosis. LaboratoriumsMedizin. 2016;39(s1). doi:10.1515/labmed-2015-0106
24. Yaylayan VA, Huyghues-Despointes A. Chemistry of Amadori rearrangement products: Analysis, synthesis, kinetics, reactions, and spectroscopic properties. Crit Rev Food Sci Nutr. 1994; 34(4):321-369. doi:10.1080/10408399409527667
25. Little RR, Roberts WL. A review of variant hemoglobins interfering with hemoglobin A1C measurement. J Diabetes Sci Technol. 2009; 3(3):446-451. doi:10.1177/193229680900300307
26. Piel FB, Patil AP, Howes RE, et al. Global distribution of the sickle cell gene and geographical confirmation of the malaria hypothesis. Nat Commun. Published online 2010:7.
27. Bachir D, Galacteros F. Hemoglobin C disease. Orphanet Encyclopedia. Published November 2004. https://www.orpha.net/data/patho/GB/uk-HbC.pdf
28. Zeng YT, Huang SZ, Ren ZR, Li HJ. Identification of Hb D-Punjab gene: application of DNA amplification in the study of abnormal hemoglobins. Am J Hum Genet. 1989; 44(6):886-889.
29. Bachir D, Galacteros F. Hemoglobin E disease. Orphanet Encyclopedia. Published November 2004. https://www.orpha.net/data/patho/GB/uk-HbE.pdf
30. Little RR, La’ulu SL, Hanson SE, Rohlfing CL, Schmidt RL. Effects of 49 different rare Hb variants on HbA1c measurement in eight methods. J Diabetes Sci Technol. 2015; 9(4):849-856. doi:10.1177/1932296815572367
31. Little RR, Rohlfing CL, Sacks DB, National Glycohemoglobin Standardization Program (NGSP) Steering Committee. Status of hemoglobin A1C measurement and goals for improvement: from chaos to order for improving diabetes care. Clin Chem. 2011; 57(2):205-214. doi:10.1373/clinchem.2010.148841
32. International Federation of Clinical Chemistry (IFCC) Standardization of HbA1c. NGSP Web Site. Accessed July 25, 2022. http://www.ngsp.org/docs/IFCCstd.pdf
33. Little RR, Rohlfing C, Sacks DB. The National Glycohemoglobin Standardization Program: Over 20 years of improving hemoglobin A1C measurement. Clin Chem. 2019; 65(7):839-848. doi:10.1373/clinchem.2018.296962
34. Finke A, Kobold U, Hoelzel W, Weykamp C, Miedema K, Jeppsson JO. Preparation of a candidate primary reference material for the international standardisation of HbA1c determinations. 1998; 36(5):299-308. doi:10.1515/CCLM.1998.051
35. Jeppsson JO, Kobold U, Barr J, et al. Approved IFCC reference method for the measurement of HbA1c in human blood. Clin Chem Lab Med. 2002; 40(1):78-89. doi:10.1515/CCLM.2002.016
36. NGSP Website. Accessed July 25, 2022. http://www.ngsp.org
37. NGSP Obtaining Certification. NGSP Web Site. Accessed August 1, 2022. http://www.ngsp.org/critsumm.asp
38. Weykamp C, John WG, Mosca A. A review of the challenge in measuring hemoglobin A1C. J Diabetes Sci Technol. 2009; 3(3):439-445. doi:10.1177/193229680900300306
39. Liu L, Hood S, Wang Y, et al. Direct enzymatic assay for %HbA1c in human whole blood samples. Clin Biochem. 2008; 41(7-8):576-583. doi:10.1016/j.clinbiochem.2008.01.013
40. Ferri S, Kim S, Tsugawa W, Sode K. Review of fructosyl amino acid oxidase engineering research: a glimpse into the future of hemoglobin A1c biosensing. J Diabetes Sci Technol Online. 2009; 3(3):585-592.
41. nstructions For Use VITROS Chemistry Products. HbA1cPub. No. J55871_EN Version 5.1.
42. More about HbA1c: Clinical Use. NGSP Web Site. Accessed July 26, 2022. http://www.ngsp.org/ADA.asp
