Detection of the hepatitis B surface antigen (HBsAg) in patients with occult hepatitis B using a sensitive HBsAg assay
Danny Wong1,2, James Fung1,2 Claire Chen3, Lung-Yi Mak2, Wai-Kay Seto, ManFung Yuen1,2
1Department of Medicine
2State Key Laboratory of Liver Research, The University of Hong Kong, Hong Kong.
3Abbott Laboratories, Singapore.
E-mail:- anurag.bhargava@abbott.com
2021 American Association for the Study of Liver Diseases (AASLD) Meeting. Nov 12-15, 2021
APFCB News Volume 1, Issue 1
Materials & Methods
HBsAg was measured by HBsAg NEXT in archived samples collected from 4 groups of patients/subjects with undetectable HBsAg by conventional assays but with serological/clinical evidence of OBI: Group 1: 200 HBsAg-negative, HBV DNA-positive blood donors. Group 2: 38 HBsAg-negative patients receiving immuno-suppressive therapy. Group 3: 800 chronic hepatitis B (CHB) patients with spontaneous HBsAg seroclearance. Group 4: 100 HBsAg-negative subjects recruited from a community project.
HBsAg was measured by HBsAg NEXT on an ARCHITECT i2000SR analyzer (Abbott Laboratories). Results were expressed as signal over cut off (S/CO). A S/CO of ≥1 was considered initial reactive. Initial reactive samples were retested in duplicate and confirmed with the NEXT Confirmatory test.
Results
Group 1: HBsAg-negative, HBV DNA-positive blood donors, 200 blood donors:
- Undetectable HBsAg by the PRISM HBsAg Assay [Abbott].
- Detectable HBV DNA results by NAT (determined by Procleix [Grifols Diagnostic]; LLOD 3.4 IU/mL), followed by confirmation by an in-house PCR assay2.
- Referred from the Hong Kong Red Cross Blood Transfusion Service to the Queen Mary Hospital, Hong Kong for clinical follow up during 2009 – 2020.
- 10/200 (5%) were confirmed positive by HBsAg NEXT. (mean S/CO 3.78 ± 1.17) An increment of 5% detection rate if HBsAg NEXT was used instead of PRISM HBsAg Assay.
Group 2: HBsAg-negative patients receiving immuno-suppressive therapy:
- 38 HBsAg-negative (determined by either ARCHITECT HBsAg Qual II or Elecsys HBsAg II [Roche]), anti-HBc-positive patients with haematological malignancies were followed up for 2 years after receiving either rituximab-containing therapy or allogeneic hematopoietic stem cell transplantation3.
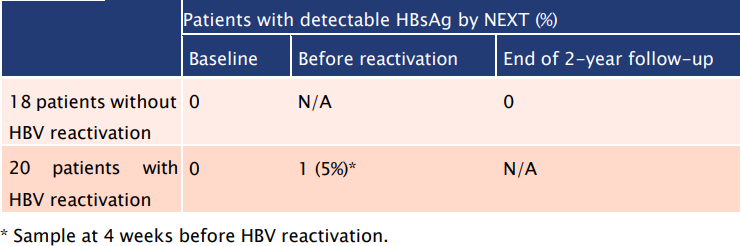
- 20 patients had HBV reactivation whereas the remaining 18 did not experience reactivation.
- 1/20 (5%) patients with reactivation had reactive HBsAg NEXT result at 4 weeks before reactivation.
Table 1: The use of HBsAg NEXT could depict HBV reactivation prior to the emergence of HBV DNA
Group 3: Patients with HBsAg seroclearance:
- 800 patients with HBsAg seroclearance determined by either ARCHITECT HBsAg Qual II [Abbott] or LIAISON XL MUREX HBsAg Assay [DiaSorin]). Samples collected 0.5 – 29.7 years (median 7.8 years) after HBsAg seroclearance.
- 59 (7.3%) had detectable HBsAg by HBsAg NEXT.
- Distribution of detectable HBsAg (by HBsAg NEXT) with sample collection time is as follows:
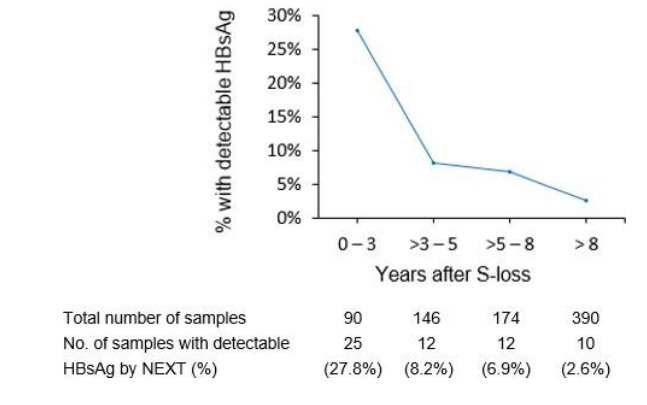
Fig 1: HBsAg NEXT detected HBsAg in 7.3% patients with HBsAg seroclearance determined by conventional assays.
Group 4: HBsAg-negative individuals from a community project:
- 100 HBsAg-negative apparently healthy subjects (determined by Elecsys HBsAg II) from a territory-wide community study4, out of which 29 (29%) were anti-HBc positive.
- 7 (7%) had HBsAg detectable by HBsAg NEXT.
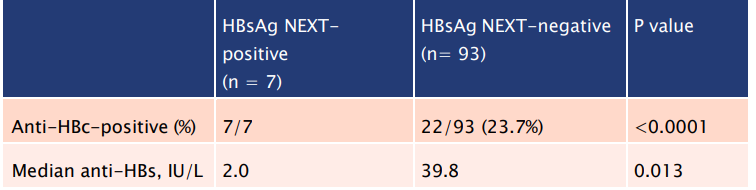
- All 7 samples with detectable HBsAg were also anti-HBc positive:
Table 2: HBsAg NEXT could detect OBI in apparently healthy HBsAg negative and antiHBcpositive subjects in the community.
Conclusion
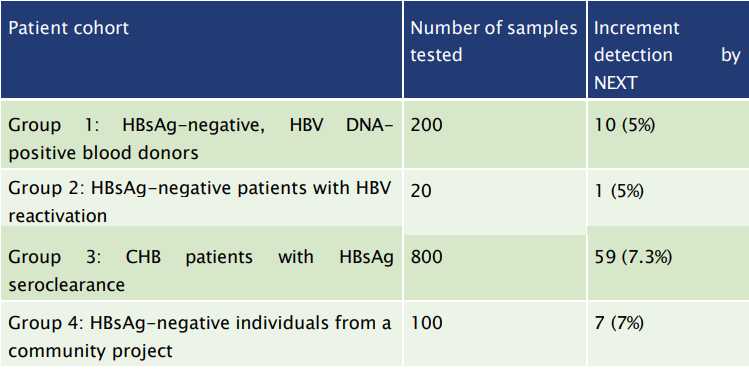
Overall, HBsAg NEXT conferred an increment of 5 – 7.3% detection rate when comparing with conventional HBsAg assays:
Table 3: HBsAg NEXT can improve the prevention of HBV transmission and HBV reactivation and allow a better policy implementation regarding the prevention of HBV-related complications.
Acknowledgements
The authors would like to thank Mr John Yuen, staff of Abbott Laboratories, The Red Cross Blood Transfusion Service, and The Hong Kong Liver Foundation for their assistance.
1. Lou S, Taylor R, Pearce S, Kuhns M, Leary T. 2018. An ultra-sensitive Abbott ARCHITECT (R) assay for the detection of hepatitis B virus surface antigen (HBsAg). J Clin Virol 105:18-25.
2. Tsoi WC, Lelie N, Lin CK. 2013. Enhanced detection of hepatitis B virus in Hong Kong blood donors after introduction of a more sensitive transcription-mediated amplification assay. Transfusion 53:2477-88.
3. Seto WK, Wong DK, Chan TY, Hwang YY, Fung J, Liu KS, Gill H, Lam YF, Cheung KS, Lie AK, Lai CL, Kwong YL, Yuen MF. 2016. Association of Hepatitis B Core-eelated antigen with Hepatitis B virus reactivation in occult viral carriers undergoing high-risk immunosuppressive therapy. Am J Gastroenterol 111:1788-1795.
4. Liu KSH, Seto WK, Lau EHY, Wong DK, Lam YF, Cheung KS, Mak LY, Ko KL, To WP, Law MWK, Wu JT, Lai CL, Yuen MF. 2019. A Territorywide Prevalence Study on Blood-Borne and Enteric Viral Hepatitis in Hong Kong. J Infect Dis 219:1924-1933.
Disclosures This study is supported by Abbott Laboratories.
Contact Information Claire Chen, Global Scientific Affairs, Core Diagnostics, Abbott Laboratories.
Email: Claire.chen@abbott.com
Danny Wong,Department of Medicine, University of Hong Kong.
Email: danywong@hku.hk
