Effectiveness of PlGF as a Point of Care Tool for the Prediction of Preeclampsia in HighRisk Pregnancies
Dr. Snehal S. Dhobale, Dr. Revathi Soundararajan, Dr. Kamini A. Rao
1 Maternal-Fetal Medicine, Senior Consultant, Milann Hospital, Bengaluru, India..
1 Maternal-Fetal Medicine, Associate Fellow, Milann Hospital, Bengaluru, India
.2 Medical Director, Milann Hospital, Bengaluru, India.
APFCB News Volume 4, Issue 2, 2025
Materials and Methods
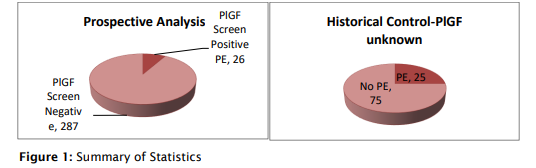
This single-center prospective observational study was conducted at Milann Hospital, Bengaluru, from April 2012 to April 2014. It enrolled 313 pregnant women, mostly high-risk (80%), with conditions like thrombophilia, hypothyroidism, autoimmune disorders like SLE, and hypertension. Participants underwent PlGF screening using (Triage®) and uterine artery Doppler studies between 20 and 24 weeks of gestation. PlGF levels below the 5th percentile were considered test-positive.5 The study aimed to evaluate the effectiveness of PlGF screening in predicting preeclampsia in this high-risk population.
Triage PlGF Percentile (pg/mL)
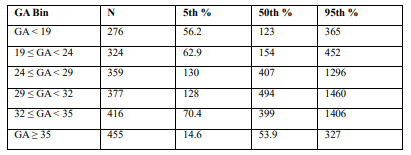
Table 1: Gestational age-based PlGF reference centiles (5th, 50th, 95th) from the Triage® POC insert (2009), which was used as a reference for risk stratification in the patients.
Participants were followed longitudinally for adverse pregnancy outcomes, including the development of preeclampsia, fetal growth restriction (FGR), and intrauterine fetal demise (IUFD) in the same lines as in the Pelican Study.6 Women who tested positive with low PlGF were subjected to enhanced surveillance protocols, which included initiation or titration of antihypertensive therapy where appropriate, serial fetal growth scans, Doppler assessments, and modified biophysical profiles, customized according to the gestational age and severity of the condition.
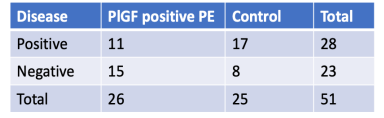
To evaluate the impact of PlGF-based risk stratification, maternal and fetal outcomes of testpositive participants were compared with those of 100 historical controls who had delivered prior to the adoption of PlGF testing at the same institution.
Results
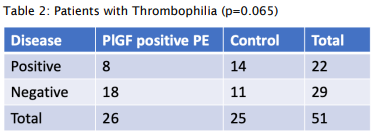
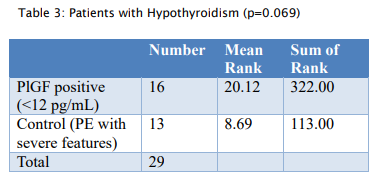
- PlGF demonstrated predictive value for preeclampsia in specific subgroups, including patients with thrombophilia (15/26; p = 0.065) and hypothyroidism (18/26; p = 0.069), which were close to significance.
- Effective lead time (the interval between test positivity and clinical diagnosis) was significantly prolonged in the PlGF-positive group, as shown by a marked difference in mean ranks (p < 0.05, Mann–Whitney test).
- Among patients who developed preeclampsia with severe features, the lead time was notably longer (p = 0.0001), offering a critical window for intervention, compared to historical controls.
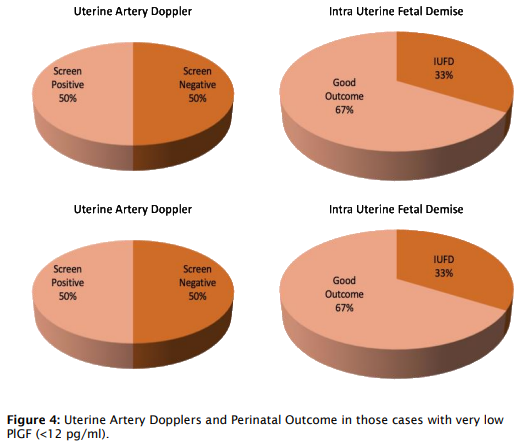
- Interestingly, 61% of PlGF-positive patients were uterine artery Doppler screen negative, highlighting PlGF’s ability to identify at-risk pregnancies that Doppler may miss.
- Among women who developed PE with severe features, those identified via PlGF testing and subjected to close surveillance delivered at higher gestational ages and had improved birth weights compared to historical controls.
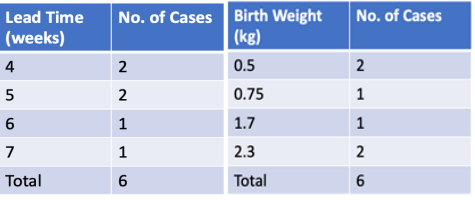
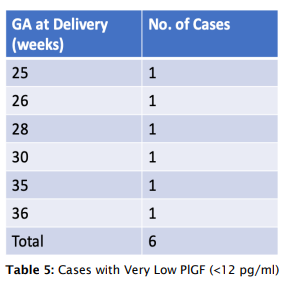
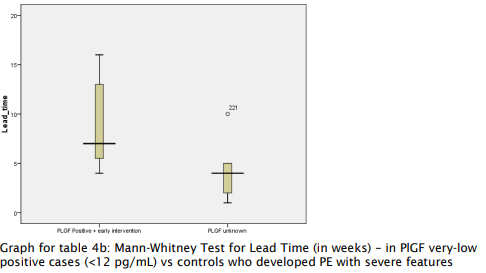
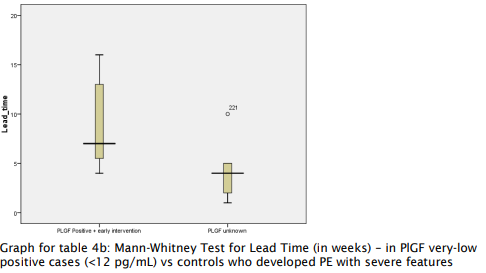
- Patients with very low PlGF levels (<12 pg/ml) showed improved outcomes following aggressive monitoring, with a lead time of 4-7 weeks, enabling delivery closer to the threshold of viability.
- However, 13.3% (4/30) of PlGF-positive patients did not develop PE, and three PlGFnegative patients developed severe PE, all of whom were carrying twin pregnancies, suggesting unique dynamics in multifetal gestation.
p=0.0001
Table 4a and 4b: Mann-Whitney Test for Lead Time
(Test to Delivery Time)
Discussion
Preeclampsia remains a significant global health challenge, and early diagnosis using reliable biomarkers is essential to improve maternal and fetal outcomes. Early diagnosis is key—but traditional tools don’t always catch it in time. Placental Growth Factor (PlGF), a novel angiogenic marker, shows promising predictive value and enhances clinical decision-making through timely surveillance and intervention. As a novel angiogenic biomarker, PlGF offers a more precise way to identify at-risk pregnancies earlier. In our study, it proved especially helpful in high-risk women, even detecting cases missed by standard Doppler scans. By enabling timely intervention and closer monitoring, PlGF testing led to better outcomes, including higher birth weights and longer gestation. It’s a step toward more proactive, personalized prenatal care.
Key Takeaways from Our Study:
- PlGF is a biomarker of placental dysfunction with reduced levels in early-onset severe preeclampsia and fetal growth restriction.
- Between 20 and 34+6 weeks, PlGF enhances diagnostic accuracy with high sensitivity (94.5%) and specificity (95%) for early-onset preeclampsia.
- PlGF testing guides decisions on surveillance and referral, improving outcomes. ? The Triage PlGF assay is more effective, enabling earlier detection and targeted surveillance when compared to historical controls who were monitored without the test.
Larger multicentric studies are needed to validate the utility of second-trimester PlGF screening and support its broader clinical implementation.
Conclusion
PlGF-POC (Triage®) testing is a powerful tool for predicting and managing preeclampsia, especially in resource-limited settings.
Acknowledgment
The investigators and authors acknowledge Dr. Gautham Pranesh for his contribution to the statistical analysis of this study.
Conflicts of Interest
The Investigators/authors declare that they have no conflicts of interest related to the conduct of the study
Presentation History
This study was presented at the ISOM–ISSHP World Congress 2014 and will resume with new research after the re-launch of the test in April 2025 in India.
- The Alere Triage PlGF POC test is currently available as Quidel Triage® PlGF Test.
- The Investigators have been tagged with the designations that they held at the time of the conduct of this study. However, currently their designation are as follows:
- Dr Snehal Dhobale Kohale 1 , Consultant Fertility Specialist and Clinical Director, Ova Fertility and Women Care, Thane, India.
- Dr Revathi Soundarajan 1*, Managing Director, Mirror Health, Bengaluru, India, Secretary, SMFM (I), Organizing Secretary, ISSHP World Congress 2023, Indian Co-Chair, PEN (I)
- Dr Kamini Rao 2, Co-Founder and Chairman, Dr Kamini Rao Hospitals, Bengaluru, India.
Authors Contribution: All authors are independent experts and equally contributed to the
expert article published.
Funding: None declared
Conflict of Interest: None declared
1. Obstetrics & Gynecology Clinics of North America. June 2010; 37(2).
2. Magee LA, Brown MA, Hall DR, Gupte S, Hennessy A, Karumanchi SA, Kenny LC, McCarthy F, Myers J, Poon LC, Rana S, Saito S, Staff AC, Tsigas E, von Dadelszen P. The 2021 International Society for the Study of Hypertension in Pregnancy classification, diagnosis & management recommendations for international practice. Pregnancy Hypertens. 2022 Mar; 27:148-169. doi: 10.1016/j.preghy.2021.09.008. Epub 2021 Oct 9. PMID: 35066406.
3. Knudsen UB, et al. Pregnancy Hypertension. 2011; 2:8–15.
4. Benton SJ, et al. Am J Obstet Gynecol. 2011;205: 469.e1–469.e8.
5. Saffer C, et al. Pregnancy Hypertension. 2013; 3:124–132.
6. Chappell LC, et al. Circulation. 2013; 128:2121–2131 (PELICAN Study).

.PNG)
.PNG)